Manufacturer for Bloodless Glucose Monitor - UBREATH ® Spirometer System (PF280) – e-Linkcare
Manufacturer for Bloodless Glucose Monitor - UBREATH ® Spirometer System (PF280) – e-Linkcare Detail:
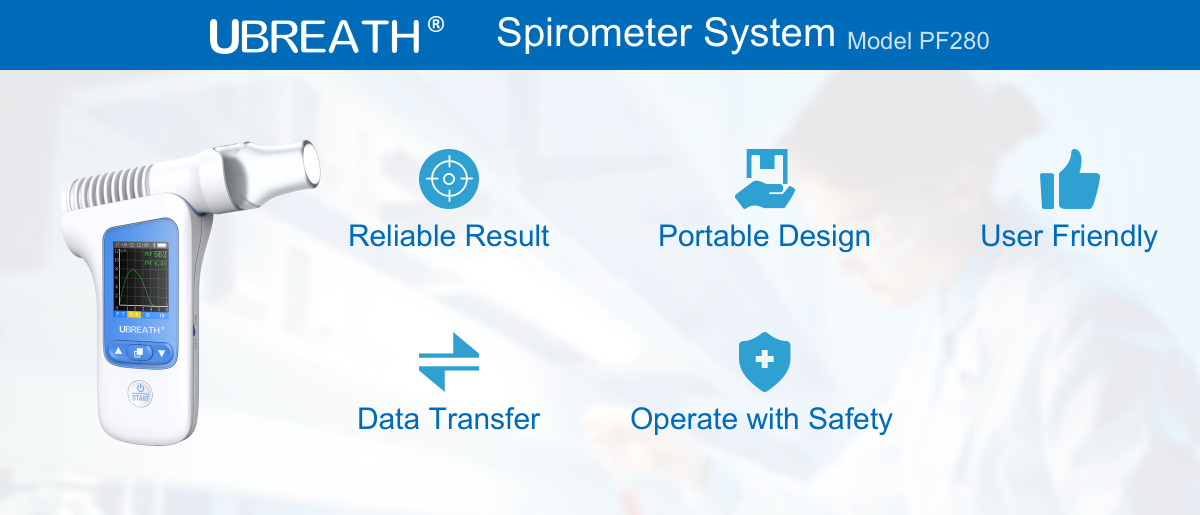
Reliable Result
Provides 6 parameters: PEF, FVC, FEV1, FEV1/FVC, EFE50, FEF75.
Accuracy and repeatability comply with ATS/ERS task force standardization (ISO26782:2009)
Complies with the ATS/ERS requirement for flow sensitivity down to 0.025L/s which is a vital characteristic for the diagnosis and monitoring of COPD patients.Portable Design
Hand-held device and easy to operation.
Automated BTPS calibration and free of environmental conditions influence.
Lightweight combines the benefits of portability.
Maintain easily and free daily Calibration.
Zero Cross-Contamination
Assured hygiene with disposable pneumotach gives NO authority to cross-contamination.
Patented design offers prevention and no daily sterilization is required.
Automated quality control and correction algorithm to minimize interference from operation.
User Friendly
Incentive graph and digital indicators displayed supports doctors’ disease quick assessment.
Colorful range indicator allows quick assessment for better visual clarity.
Easily connect to PC for data exchange.
NO daily Calibration and Sterilization required.
Data Transfer
Easily connect to PC via dedicated Bluetooth Communication Module for data exchange.
Access to UBREATH Software for more data analysis function.
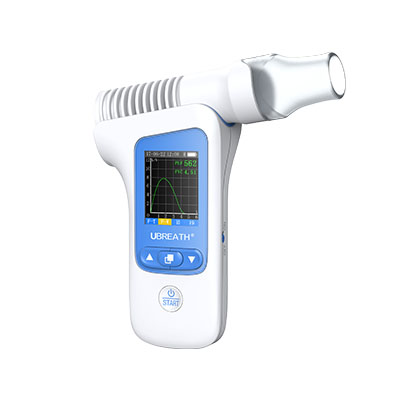
UBREATH Spirometer System(Model No. PF280)is a high-quality, easy to use, portable spirometer that delivers an ideal combination of portability, accuracy and safety. And it also helps doctor analyze the pulmonary data through V-T/F-V curve and digital indicator which is an ideal solution for the primary care, point of care, patients self-monitoring environment.

Technical Specifications
|
Feature |
Specification |
| Model | PF280 |
| Parameter | PEF, FVC, FEV1, FEV1/FVC, FEF50, FEF75 |
| Flow Detection Principle | Pneumotachograph |
| Volume Range | Volume: 0.5-8 LFlow: 0-14 L/s |
| Performance Standard | ATS/ERS 2005 & ISO 26783:2009, ISO 23747:2015 |
| Volume Accuracy | ±3% or ±0.050L |
| Power Supply | 3.7 V lithium battery |
| Battery Life | Approximately 500 complete charge cycles |
| Printer | External Bluetooth printer |
| Memory | 495 records |
| Operating Temperature | 10℃ - 40℃ |
| Operating Relative Humidity | ≤ 80% |
| Size | Spirometer: 133x76x39 mm |
| Weight | 135g (including the Flow Transducer) |
Product detail pictures:
Related Product Guide:
Persisting in "High quality, Prompt Delivery, Competitive Price", we have established long-term cooperation with clients from both overseas and domestically and get new and old clients' high comments for Manufacturer for Bloodless Glucose Monitor - UBREATH ® Spirometer System (PF280) – e-Linkcare , The product will supply to all over the world, such as: Rio de Janeiro, Malaysia, Ecuador, We'd like to invite customers from abroad to discuss business with us. We can provide our clients with high quality products and excellent service. We are sure that we will have good cooperative relationships and make a brilliant future for both parties.
We are old friends, the company's product quality has been always very good and this time the price is also very cheap.



